research and publications
Preclinical studies
Studies show that Q-actin reduces LPS-induced pro-inflammatory cytokine tumour necrosis factor alpha (TNFα) in both ex vivo human serum and THP-1 cells. TNFα can drive degenerative changes such as in joints when chronically elevated. Research shows that ido-BR1 works in a dose-dependent manner to reduce inflammatory markers, including LPS-induced production of TNFα, IL-6, nitric oxide and the transcription factor NF-κB.
Clinical studies
>> Q-actin Joint Function and Mobility
A recent clinical trial, published in Current Rheumatology Reviews, investigated the effects of Q-actin™ on joint health in 91 adults with moderate joint issues. The study aimed to compare the effectiveness of Q-actin™ to a placebo. Out of the initial 101 participants enrolled, 91 were included in the analysis.
The participants were divided into three groups: one group received a placebo, while the other two groups received daily doses of either 20 mg or 100 mg of Q-actin™ for a duration of six months. Throughout the study, the participants’ joint function and mobility were evaluated at 30-day intervals using three established and reliable assessment tools: the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), the Visual Analogue Scale (VAS), and Lequesne’s Functional Index (LFI).
The results showed that both groups receiving Q-actin™ experienced significant improvements in joint function and mobility, as well as reductions in pain, compared to the placebo group. These positive effects were observed consistently at each evaluation point during the study, as assessed by all three evaluation methods (WOMAC, VAS, and LFI). Furthermore, the study indicated that the benefits of Q-actin™ were dose-dependent, meaning that higher doses resulted in more noticeable improvements in joint health.
Overall, the findings of this clinical trial suggest that Q-actin™ has the potential to significantly enhance joint function and alleviate pain in individuals with moderate joint health issues.
>> Q-actin Outperforms Glucosamine - Chondroitin
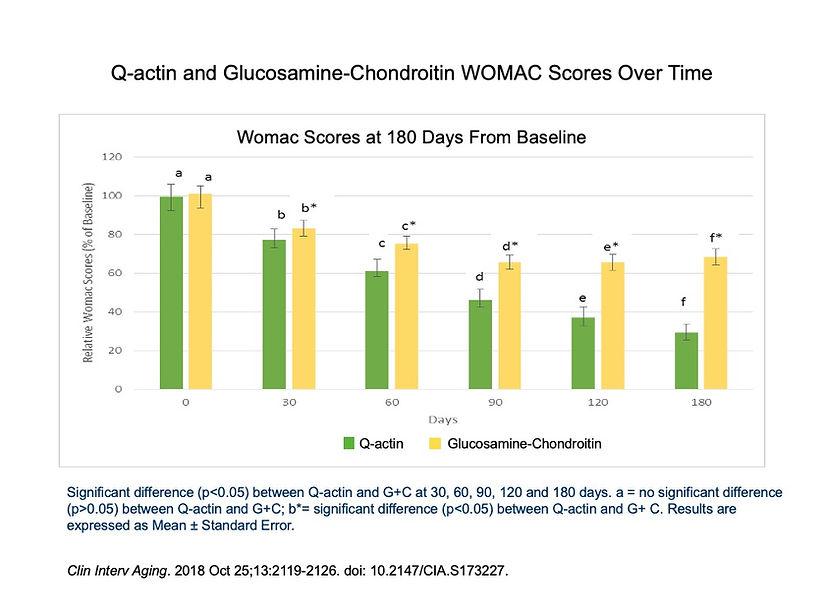
A clinical study, which was randomized and double-blinded, was published in the journal Clinical Interventions in Aging. The study aimed to compare the effectiveness of Q-actin and glucosamine-chondroitin in relieving knee discomfort and improving mobility in individuals with moderate joint health issues associated with aging.
The study included 122 men and women aged 40 to 75 from three different countries. Participants were randomly assigned to two groups: one group consumed 1,350 mg of glucosamine-chondroitin, and the other group consumed 10 mg of Q-actin, both taken twice daily for a duration of six months.
At various intervals (30, 60, 90, 120, and 180 days), the participants were evaluated using three well-established assessment tools: WOMAC, VAS, and LFI. The results indicated that individuals who consumed Q-actin experienced significant improvements in joint health at each evaluation point compared to those who took glucosamine-chondroitin.
This study provides evidence for the efficacy of Q-actin in relieving knee discomfort and enhancing mobility, surpassing the effectiveness of glucosamine-chondroitin. The findings suggest that Q-actin has the potential to restore, maintain, and protect joint health, which is particularly important for older adults, as well as elite and recreational athletes. Additionally, it may be beneficial for individuals dealing with weight-related issues.
publications
Current Rheumatology Reviews
March 2023
Effectiveness of Cucumis sativus Extract Versus Glucosamine-Chondroitin in the Management of Moderate Osteoarthritis: a Randomized Controlled Trial
Clinical Interventions in Aging
October 2018